What Type of Bonding Is Present in Iodine Molecules
A London-dispersion forces b ion-dipole interactions c ionic bonding d dipole-dipole attraction e covalent-dipole interactions. What is the predominant intermolecular force in CBr4.

If3 Molecular Geometry Science Education And Tutorials
A London-dispersion forces b.

. Hydrocarbons that contain only carbon-to-carbon single bonds are called alkanes. What is the major attractive force that exists among different I2 molecules in the solid. Hydrocarbons containing at least one carbon-to-carbon double bond are called alkenes and compounds with at least one carbon-to-carbon triple bond.
These are also referred to as saturated molecules. Another means of classification depends on the type of bonding that exists between carbons. Elemental iodine I2 is a solid at room temperature.

Trigonal Planar Molecular Geometry Molecular Geometry Molecular Shapes Chemistry Projects
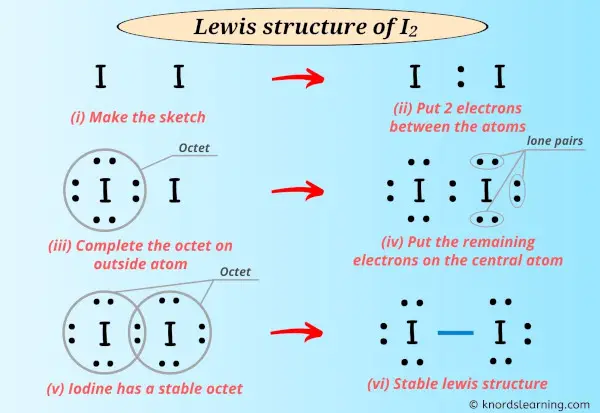
No comments for "What Type of Bonding Is Present in Iodine Molecules"
Post a Comment